100 Torri
Molte pagine di questo sito sono dotate di commento musicale, accendete le casse del computer!
L'AGGIORNAMENTO DI QUESTO SITO SARÀ RIPRESO AL PIÙ PRESTO
![]()
|
|
È stata fino al 2009 una antica
istituzione ospitata dal Circolo Cittadino di Ascoli
Piceno. Si è chiusa quando quest'ultimo sodalizio ha
recuperato, colpevolmente, i locali, espellendola,
mentre il Presidente del Circolo di scacchi, Luciano
Morganti, che ne aveva creato la ripresa, si è
trasferito all'estero. Confidiamo che l'associazione
possa riattivarsi nel prossimo futuro. |
![]()
|
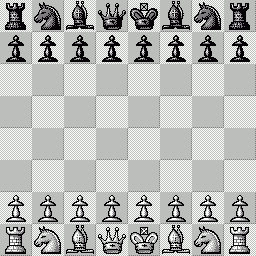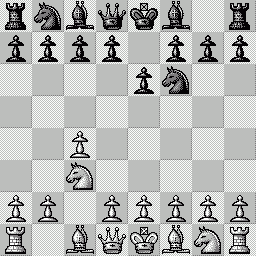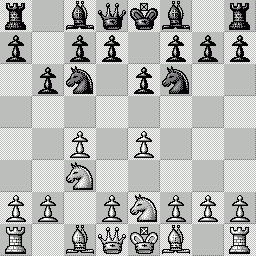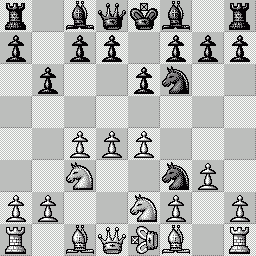
LA PARTITA ANIMATA SCELTA PER VOI Questa "miniatura"
è stata giocata nel 1979 fra Mantia (Bianco) e
Trogdon (Nero). Per le precedenti partite animate, clicca qui |
|
![]()
|
|
|
|||||
![]()
|
|
|
![]()
SCACCHIERE E CHIACCHIERE
 Il presidente Franklin al tavolo di scacchi |
Ben 24 dei 43 Presidenti degli Stati
Uniti in carica erano discreti giocatori di scacchi.
Da Washington ad Obama (è il caso di dire: Bianchi e
Neri), passando per Jefferson, Lincoln, i due
Roosvelt, Truman, Eisenhauer, Kennedy, Carter e
Billy Clinton: tutti hanno teso le loro trappole
sulla scacchiera politica... |
|
Nella pittoresca
cittadina slovacca di Banska Stiavnica si è tenuta
recentemente la tradizionale partita di scacchi
viventi, con personaggi vestiti di pittoreschi
costumi medioevali, armati con spadoni e scudi. |
|
|
|
È il momento dei giovani italiani
nelle gare mondiali di scacchi. Nel 50° campionato
juniores, tenutosi nell'agosto scorso in India, Axel
Rombaldoni (della famiglia pesarese con 5 grandi
giocatori) ha conseguito il titolo di Grande
Maestro. Dopo 6 partite era imbattuto con 6
vittorie. Fabiano Caruana ha vinto il torneo AAI
International con un punto intero di vantaggio sul
secondo. Ha guadagnato 11 punti Elo, portandosi a
2712. |
|
|
|